PRINCETON, N.J., Sept. 17, 2024 /CNW/ – Medunik USA, member of Duchesnay Pharmaceutical Group (DPG), one of the few anchor companies selected by the Government of Canada for its Global Hypergrowth Project, continues to increase Pheburane® coverage with three more states adding Pheburane® to their Medicaid Preferred Drug lists, namely Florida, Louisiana, and Maine, with Florida being one of the top states in terms of Medicaid spending on Urea Cycle Disorder (UCD) treatments. Thus, Pheburane® is now on the Preferred Drug list in 14 states and on the Contract Drugs list in California. This major achievement allows a greater number of patients with UCD to access this important treatment option through Medicaid programs.
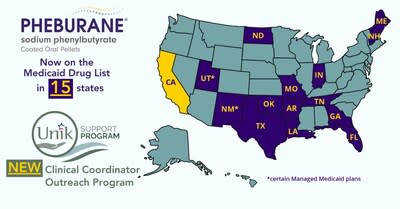

Pheburane coverage expansion in the US. (CNW Group/Medunik USA)
Pheburane® is the safe, effective and palatable adjunctive therapy to the standard of care, which includes dietary management, for the treatment of certain UCDs. Pheburane® is an innovative formulation of sodium phenylbutyrate consisting of very small coated oral pellets and is indicated for both adult and pediatric patients. Pheburane® is not indicated for the treatment of acute hyperammonemia. The most common side effects associated with sodium phenylbutyrate are menstrual dysfunction, decreased appetite, body odor and bad taste or taste aversion.1
In addition to increasing the accessibility of Pheburane®, Medunik USA has expanded its UNIK Support program with the launch of a new Clinical Coordinator Outreach Program for patients and caregivers. This adds to our existing support services including our Patient Care Liaisons, Copay programs, and Mail Order Pharmacy Services. Under the new Outreach program, patients treated with Pheburane® and their caregivers can receive personalized guidance and support.
“The new Clinical Coordinator Outreach Program brings our patient support services to the next level by offering support customized to each unique UCD patient,” said Michael Gallo, Vice President, Regulatory & Medical Affairs, Duchesnay Pharmaceutical Group. “We are resolute in the belief that patients with rare diseases deserve no less support than those suffering from more common conditions. We strive to continuously enhance the services available to these patients whose needs are often being neglected.”
Information about the new Clinical Coordinator Outreach program and the enrolling process is available at .
UCDs are rare, chronic, genetic conditions that can be fatal if left untreated, and can impact children from the time of birth. UCDs disrupt the body’s urea cycle, and therefore, the body is unable to remove the dangerous buildup of toxic chemicals, particularly ammonia, that are created from the digestion of protein. One in 35,000 people in the United States or about 28 per one million residents suffer from UCDs of different levels of severity.2
About urea cycle disorders (UCDs)
The main purpose of the Urea Cycle is to eliminate toxic ammonia from the blood and make urea, which is then excreted as urine. UCDs are rare genetic disorders that cause errors in this process, allowing high levels of ammonia, the key marker for UCDs, to build up in the bloodstream, potentially to dangerous and fatal levels. Ammonia is extremely toxic, particularly to the central nervous system. UCDs can cause catastrophic illness in newborns within 36 to 48 hours of birth despite the infants appearing normal, so they can be discharged from hospital before signs of UCDs develop. UCDs require lifelong monitoring and treatment.2
About Pheburane®
Pheburane® is a taste-masked oral formulation of sodium phenylbutyrate, approved by the Food and Drug Administration as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients with UCDs, involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS). Pheburane® is not indicated for the treatment of acute hyperammonemia.1
Pheburane®, developed by Lucane Pharma, is under exclusive distribution in the U.S. through Medunik USA. For further information, visit Pheburane.com.
INDICATION AND IMPORTANT SAFETY INFORMATION
What is Pheburane®?
Pheburane® is a prescription medicine, used along with a specific diet, for the long-term management of adults and children with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinate synthetase (AS).
Episodes of sudden, rapid increase of ammonia in the blood (acute hyperammonemia) may happen in people during treatment with Pheburane®. Pheburane® is not used for the treatment of acute hyperammonemia, which can be life-threatening and requires emergency medical treatment.
Before taking Pheburane®, tell your healthcare provider about all of your medical conditions, including if you:
have heart problems.
have kidney or liver problems.
have diabetes (Pheburane® contains sucrose), or have a history of fructose intolerance, glucose-galactose malabsorption, or sucrose-isomaltase insufficiency.
are pregnant or plan to become pregnant. It is not known if Pheburane® will harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if Pheburane® passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby during treatment with Pheburane®.
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Certain medicines may increase the level of ammonia in your blood or cause serious side effects when taken during treatment with Pheburane®. Especially tell your healthcare provider if you or your child take:
corticosteroids
valproic acid
haloperidol
probenecid
Know the medicines you take. Keep a list of them to show your or your child’s healthcare provider and pharmacist when you get a new medicine.
What are the possible side effects of Pheburane®?
Pheburane® can cause serious side effects, including:
Nervous system problems (neurotoxicity). Call your healthcare provider right away if you get any of the following symptoms during treatment with Pheburane®:
sleepiness
tiredness
lightheadedness
vomiting
nausea
headache
confusion
Low potassium levels in your blood (hypokalemia). Your healthcare provider will monitor your blood potassium levels during treatment with PHEBURANE and treat if needed.
Conditions related to swelling (edema). Pheburane® contains salt (sodium), which can cause swelling from salt and water retention. Your healthcare provider will decide if PHEBURANE is right for you if you have certain medical conditions that can cause swelling, such as heart failure, liver problems or kidney problems.
The most common side effects of Pheburane® include:
Your healthcare provider may do certain blood tests to check you or your child for side effects during treatment with Pheburane®.
These are not all the possible side effects of Pheburane®.
Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please read the Full Prescribing Information and Patient Information at Pheburane.com.
About Medunik USA
Based in Princeton, New Jersey, Medunik USA is part of Duchesnay Pharmaceutical Group and works to improve the health and quality of life of Americans living with rare diseases by making orphan drug therapies available in the United States. Through its strategic partnerships, Medunik USA develops and provides Americans suffering from rare disease with access to orphan drugs that are not currently available in the U.S. Medunik USA makes critical medications to treat rare diseases available to American patients who might not otherwise have access to these medications. For more information, visit www.medunikusa.com.
About Duchesnay Pharmaceutical Group
Duchesnay Pharmaceutical Group (DPG), with its affiliated companies, is headquartered in Blainville, Quebec. The Group consists of six pharmaceutical companies to meet the needs of patients in Canada, the U.S. and abroad.
DPG is one of the 8 companies across the country chosen to participate in the Government of Canada’s Global Hypergrowth Project. This appointment offers exclusive and personalized support for at least 2 years, in order to accelerate our growth to become an anchor firm in the Canadian economy.
Duchesnay Pharmaceutical Group, through its proprietary research and development, and exclusive partnerships, offers innovative treatments for a variety of medical conditions in women’s health, urology, oncology and for rare diseases, plus lower-cost generic medications. DPG recognizes the dedication and professionalism of its employees and promotes a positive culture and flexible work environment. It is deeply committed to environmental responsibility and to giving back to the community through the support of various charitable organizations.
For more information, please visit https://duchesnaypharmaceuticalgroup.com/en .
References
Pheburane® (sodium phenylbutyrate) oral pellets [Prescribing Information]. Medunik USA, Inc.
Cleveland Clinic, Urea Cycle Disorder, https://my.clevelandclinic.org/health/diseases/23470-urea-cycle-disorder


Medunik USA Logo (CNW Group/Medunik USA)
Cision
View original content to download multimedia:https://www.prnewswire.com/news-releases/medunik-usa-expands-medicaid-coverage-and-patient-support-services-for-pheburane-sodium-phenylbutyrate-302249624.html
SOURCE Medunik USA
Cision
View original content to download multimedia:
Source link : http://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=66e96f4c91294a37b2c20ca47af958cc&url=https%3A%2F%2Ffinance.yahoo.com%2Fnews%2Fmedunik-usa-expands-medicaid-coverage-113200091.html&c=16127229580713531244&mkt=en-us
Author :
Publish date : 2024-09-17 00:33:00
Copyright for syndicated content belongs to the linked Source.



